Written by: Jan Mrázek
If you follow my work, I often use my resin 3D printers to produce soft silicone moulds or pieces (original blog post and a follow-up). Resin printers can create precise and detailed patterns. You can quickly prototype and cast miniatures, dice, chocolate moulds… There are plenty of uses for soft silicone moulds. However, some silicones play well with resin-printed patterns, and some don’t. In this post, I will explain which silicones cause the trouble, why we care, and how to prevent the cure inhibition. The recipe I give you is surprisingly easy and requires no special equipment. It outperforms existing commercial solutions (e.g., Inhibit X) in price and performance.



Types of Silicone Rubber
Two types of silicone rubber are used to make a mould: tin-cure and platinum-cure silicones.
The tin-cure silicones (often called condensation) cure fine in resin-printed moulds. I’ve been using these silicones for manufacturing my resin spouts and moulds. They cure by mixing a small curing agent (usually 2-3 % by weight). The curing agent contains salts of heavy metals that trigger the curing reaction. Once you mix it, it will happily cure. No matter if it is underwater, in a resin printer mould, or cold or hot. What matters is how much curing agent you add. If you add more, it cures faster. However, the resulting mechanical properties are worse (the rubber is more fragile and can be easily torn).
The second type is platinum-cure silicones. These silicones are often mixed in a 1:1 ratio of both compounds. Sometimes, they are combined in a 10:1 ratio. This type of silicone is susceptible to the curing environment. It doesn’t cure if it is in contact with resin-printed mould, tin-cure silicone, sulphur, latex, acetone, garlic, chlorine, duct tape, and more (see, e.g., this page for an extensive list). The inhibition, often called “poisoning the silicone”, is permanent. The silicone will never cure in the presence of the substance. The near surrounding (from 0.1 mm to a couple of millimetres) of the poisonous instance stays liquid and never cures. It doesn’t even turn into a gel.
You are probably wondering – why would anyone use platinum-cured silicones when they are so much trouble to work with? Their properties are far superior to tin-cure silicones. And if you use commercially made silicone equipment, it is probably platinum-cure silicone. I will use Lukopren 5221 (tin-cure) and GMS A30 (platinum-cure) to compare the properties.
First, they are much more potent (2 MPa vs 9 MPa), much more challenging to tear and often have a much longer elongation at break (150 % vs 500 %). When we talk about mould-making, platinum-cured silicones often have immeasurable shrinkage (though GMS A30 is an exception), and tin-cured silicones usually shrink up to 2 % by volume. Platinum-cured silicones also do not degrade under UV light, and they don’t degrade with time. Since they are mixed in a 1:1 ratio, a static-mixing nozzle can combine them on the fly. Platinum-cure silicones also often have much lower viscosity.
So there are a lot of reasons to use them, however, how to use them with resin-printed molds?
What Causes the Inhibition and How to Prevent It?
Let’s start with a disclaimer – I am no chemist, and I understand it far, far worse than anything else. If I say something wrong or misleading here, please let me know in the comments! I am happy to learn and fix the text accordingly.
When you google silicone cure-inhibition or poisoning, you find a lot of Reddit threads, YouTube videos, and texts that suggest the only solution – coat your printed model in some varnish. And be sure it is not a varnish that also poisons silicone. This solution, however, is unacceptable to me. I use my moulds to make precise and detailed parts. Putting a varnish or paint is not only laborious, but it also changes the dimension significantly. It also gets stuck in low spots and removes details. Users are trying to find another recipe but without much luck.
When you try even harder or have access to scientific journals, you can find several scientific papers about the topic. The most interesting (and the only one publicly available) is PDMS Curing Inhibition on 3D-Printed Molds: Why? Also, How to Avoid It? This paper claims that the inhibition’s cause is the resin’s photoinitiator. Therefore, to prevent the cure inhibition, you must eliminate it (at least from the surface). I have heard from multiple sources (including some manufacturers) that the source of the inhibition is not the photoinitiator but traces of sulphur in the resin that are left from the manufacturing process. I have no way of finding where is the truth.
Nevertheless, the paper above suggests two recipes: the first is to cure for 4 hours and then keep the printed pieces heated to 60°C for 48 hours, or keep the printed piece at 120°C for 2-8 hours. This is rather extreme and has several downsides. The first method works only on half of the resins they tried (and also, none of them is for LCD printers); the second method is unusable for large moulds as 120°C is far beyond softening of most resins (most resins soften around 60°C). The authors don’t mind as they design moulds for tiny and beefy microfluidics. However, for large moulds, we are out of luck (unless we use Siraya Tech Sculp or Sculp Ultra – but I haven’t tested if this method works due to its difficulty to execute).
There is also a chemistry blog that claims to solve the problem by long washing times. How long? They succeed after 48 hours of washing in a stirred bath of IPA or ethanol. Also, they always use a virgin bath and change it 2 times during the procedure. This is expensive (we need a fresh bath every time) and time-consuming, but it also presents a problem with large moulds, as most resins soften when soaked in IPA for extensive periods.
Those were the DIY-feasible paths. Some papers suggest oxygen plasma treatment, coating in metal using magnetron vapour spattering, and more. This is not the way to go unless you are Ben from Applied Science.
Therefore, I dig into a lot of experiments and tweaking. But I finally found a relatively simple and cheap recipe to post-process the moulds to prevent the cure inhibition. If you want to learn more about this path, don’t leave after learning the recipe; there’s a section showing all approaches and dead ends I tried.
My Recipe Fore Platinum-cure Silicone Inhibition
I tested the recipe I presented with most of the Siraya Tech Resins (Fast, Sculp, Tenacious, Blu, Fast + Tenacious Mixtures, and Simple). I print the parts standardly and then:
- I wash them properly in two baths of IPA (just like I always do): first is a dirty bath to wash most of the resin, then a clean bath. I use Mercury X, and each cycle lasts about 10 minutes.
- Then I do the third round of cleaning – this time in soap water. I rinse the prints properly. However, I had plenty of success without this step. It just ensures there are no uncured resin residua.
- Then there are two recipes:
- For any models (require a coating solution):
- I cure the pieces for 30 minutes in Mercury X underwater. Without water, the process doesn’t work.
- I quickly dip dried and cured pieces into a 1% solution of polymethyl methacrylate (PMMA) in acetone. Once they dry, I repeat the dip. If you don’t know what PMMA is, it is acrylic, often called plexiglass. If you have a complex model, I suggest using a less concentrated solution with more dips to prevent residue deposition on the surface where the acetone got stuck.
- For thick-walled models (requires no extra equipment):
- I cure the pieces for 30 minutes in Mercury X underwater.
- I let them sit for at least 6 hours in the water
- I change the water and cure them for another 30 minutes.
- No coating in PMMA is necessary.
- For any models (require a coating solution):
That’s it. Now, your printed parts are ready to be used for casting platinum-cured silicone!
Why this recipe? I noticed that there has to be some interesting reaction (that I don’t fully understand) when curing underwater that prevents silicone poisoning. Air oxygen inhibits the curing reaction of the resin (which is hopefully a well-known fact). Initially, I thought that underwater curing is adequate as it prevents oxygen from reaching the surface of the prints. Therefore, no oxygen can reach the surface, and the curing reaction that disarms the photoinitiator also happens on the surface. However, I no longer think so. I tried using glycerol instead of water (which has the advantage of not being soaked by resin). The prints were cured nicely (much better than on-air) but poisoned the silicone. After consultation with a few people, I think that the photoinitiator (or its residual products) dissolves in water when illuminated with UV, and thus, it is washed away. I suspect this is also the case as the water, after curing, gets a weird rubbery and unpleasant smell.
Why are there two paths for thin and thick-walled models? It seems that thoroughly washing the photoinitiator takes some time. However, resins soak water (see details); therefore, when you have a thin-walled model, it absorbs the moisture quickly, softens, and gets deformed. Thick-walled models don’t lose structural integrity. Consequently, we can wash them properly. For thin-walled models, we try to eliminate as much of the photoinitiator as possible. For the rest, we try to seal it in a thin coating of PMMA that should prevent any photoinitiator from leaking to the surface. I had no success, only with PMMA. I guess that the PMMA coating solution dissolves the photoinitiator on the surface and mixes it with it. Therefore, if no elimination is performed, we get the photoinitiator (or its residue) all over the surface. However, if we remove it from the surface, we can seal it. Without sealing, it seems that the photoinitiator (or its residue) leaks to the surface again over time. However, this is no longer true if we apply the long enough washing. The PMMA creates an immeasurably thin coating, and the printed pieces have a nice shiny surface.


To prepare the coating solution, take a piece of (preferably clear) plexiglass and dissolve it in acetone. It takes about 1 hour to dissolve a piece. Note that there are better solvents for plexiglass (e.g., dichloromethane), but they are much harder to obtain.



Results
The results are more than satisfactory. I tested my recipe with the GMS A series of silicones and the SmoothOn Mold Star series. Post-processing adds about 45 minutes/5 hours (based on your chosen path) compared to traditional post-processing, which is bearable. The patterns never go through any stress in the coating paths, preserving dimensional stability. Some stress might be added in the slow path since the resin soaks some water. However, the coating of PMMA is easy to do, and it does not disturb the surface in any way. You can see the model’s individual voxels imprinted in the silicone! This is a vast improvement over spray painting.






The process is, however, not 100% reliable. There are some free variables that I haven’t been able to identify and eliminate. Roughly in 1 out of 20 attempts, the silicone gets poisoned. If this happens, I rerun the process and have succeeded so far. Therefore, there is some aspect of the process I do not have under control and I am unaware of its impact. Possibly ambient temperature? Humidity? I am not able to say at the moment. For this reason, I always try silicone on a small part of the model whenever I have a new mould. Cleaning uncured silicone is annoying.
The treatment also seems to be persistent. I have over 60 pulls from some of my moulds without any need to repeat the process. Also, moulds treated with PMMA do not need any mould release. The silicone does not stick to them.
The process doesn’t need any special equipment or hard-to-get or expensive chemicals.
One interesting finding is that PMMA bonds well to the surface and gives it a nice finish. It also seems to improve the nail-scratch resistance somewhat. Note that these are only my impressions during the experiments. I haven’t explored this aspect of the coating yet, and I have no complex data on this.



Hey, and What About InhibitX? Or Release Agents?
In one of my previous blog posts, I linked to Joshua’s blog post about preventing cure-inhibition. His solution was to use InhibitX. While finding a working recipe, I also got a bottle of it. It is a pretty expensive substance (and also challenging) – 300 ml costs about 70 €. You don’t use much of it, but it’s still expensive (especially when you spill it).
InhibitX works, but it does not work on its own. Joshua heat treats the printed parts before soaking them in InhibitX. I also found that without heating them (though I do not do it as aggressively as Joshua), InhibitX doesn’t work. However, InhibitX has one annoying issue. The silicone sticks well to surfaces treated by it. Therefore, if you don’t apply a release agent or don’t apply it properly, you can get ruined casting and mould. The silicone is rugged to get off. This is where the PMMA coating shines – the silicone doesn’t bond.
This brings us to the usage of release agents. I have found that paraffine or vax-based release agents don’t prevent poisoning the silicone (e.g., Easy Release 200, Luporen separátor, and others). What somewhat works are PVA-based separators. However, they are hard to apply correctly without leaving visible trace marks. PVA is also hard to use on resin moulds as it doesn’t soak the surface. What helps a little is to add a drop of soap to it. The PVA-based release agents also tend to fill the low spots and lose details. I also encountered problems with sharp edges – the separator did not stick there, and the sharp edges caused inhibition. Lastly, you have to wash and reapply the coating before every use. It’s not worth the time and effort, in my opinion.
PS: Some people are claiming that InhibitX is just naptha (according to the safety sheet). However, I don’t believe this is true. Naptha is only a solvent for the active ingredient, which is probably harmless and, therefore, is not listed on the safety sheet. I base my observation on the bottle saying, “Shake well before use” (if it was only naphtha, why mix it?), and the liquid also has some brown tint. Also, once it dries on a surface, it leaves brownish stains. I also did experiments with naphtha and other solvents, which showed they do not prevent inhibition.
The Path Towards the Recipe
The path toward finding the recipe was much longer than I anticipated. I had so many partial successes that, however, were dead ends. Usually, the procedure worked for flat surfaces, but still, it caused inhibition in sharp inner corners.
I used a triangular staircase model for my test (see photos below). This model is small enough not to waste too much material and contains details and inner corners. The internal corners proved crucial for the experiments as they caused most of the troubles. Some methods worked well on external surfaces. However, the internal corners suffered.
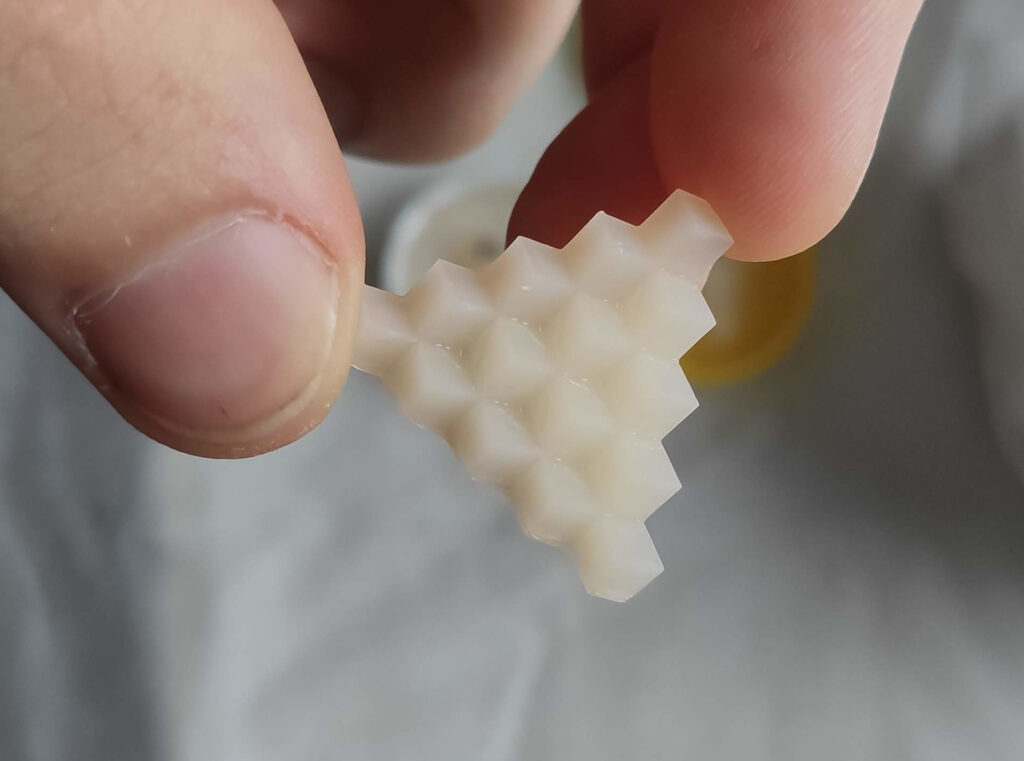










First, I experimented with curing the models. I tried curing them for an extended period, using summer sunlight and curing them warm and cold. I even cured them locally with a high-power LED. I also tried various wavelengths from 460-304 nm. None of it gave promising results.
I tried various cleaning methods, from soap, ultrasonic cleaner, and different solvents (ethanol, acetone, xylene, naphtha, …). Also, none of them seem to be 100% working.
Washing the prints in sodium hydroxide gave interesting results. Based on the length of washing, the cure inhibition was either more-less prevented or started again. It seems like in short washing cycles (10 % solution for 10-15 minutes) it gets rid of the photoinitiator. However, washing it longer again starts poisoning the reaction again. This might be because NaOH damages the resin (as confirmed by the guys from Siraya Tech), causing more poisonous compounds to be brought to the surface. Interestingly, washing in a 20 % citric acid solution has a similar effect.
Conclusion
Although I showed you the relatively simple procedure for preventing the cure inhibition, it is still some extra work, and it is up to you to decide if it is worth it. Using tin-cure silicone might be worth it if you make a mould for a single or only a few pulls. They don’t require any post-processing (they even cure in just cleaned and cured mould) and work just fine – after all, I used them for the last two years for all the mould making.
However, the process is worth it if you require a long-lasting or robust mould. The search for the recipe started after I released my resin tank cleaning kits. If you don’t know them, check the video below:
So far, I made them from tin-cured silicones. However, starting in January 2022, all my spouts will be made using high-quality platinum-cured silicone. Therefore, the spouts and spatulas should last indefinitely. This is also why the kits were unavailable for a few weeks – I was switching the process. Also note that I extended those kits for new printers: Mars 1 and Mars 3. You can check them out in my store.
Recent news: My open letter to the 3D-printing community
I love the 3D-printing community, but I think there is room for improvement. Let’s get better in 2023! Read the full letter.
Please support my work!
If you like my work (these blog posts, my software and CAD models) and would like to see more posts on various topics coming, consider supporting me in multiple ways:
- You can become my sponsor on Github.
- If you prefer, you can also become my Patreon.
- You can buy me a coffee on Ko-fi,
- or you can purchase something from my Tindie store (also see below),
- Or you can share my work!
If you are interested in knowing what I am up to and recent sneak-peaks, consider following me on social media (Twitter, Instagram,